CONTROL OF MITOTIC FIDELITY
C. elegans zygote

GFP::tubulin, mCherry::Histone
During mitosis, it is critical that duplicated chromosomes are equally partitioned between the two daughter cells. A mechanism known as the spindle assembly checkpoint ensures that cells spend enough time in mitosis until all chromosomes have attached to microtubules of the mitotic spindle. However, it is well known that the spindle assembly checkpoint does not play a significant role in regulating mitotic duration in embryonic cell cycles, indicating that there are other mechanisms at play.
The major regulator of mitotic duration is the anaphase-promoting complex / cyclosome (APC/C), a E3 ubiquitin ligase that targets several proteins for proteolytic degradation at the end of mitosis. The APC/C is activated by a co-activator called Cdc20. We showed that phosphorylation of Cdc20 by Cdk-dependent kinases is critical to maintain the APC/C inhibited and therefore to determine the minimal duration of mitosis in embryonic divisions. On the other hand, Cdc20 dephosphorylation by protein phoshatases is the key switch that promotes mitotic exit. However, how is Cdc20 phosphorylation and dephosphorylation regulated by cell cycle cues is currently unclear.
While a minimal mitotic duration is important to prevent chromosome segregation errors, an increase in mitotic duration can, surprisingly, have detrimental consequences for embryonic viability. Indeed, extending mitotic duration can result in cell cycle arrest and embryonic lethality. Therefore, a big challenge of mitosis is balancing the need to ensuring accurate chromosome segregation while minimizing the time spent in a vulnerable cell cycle phase.
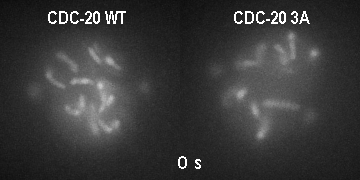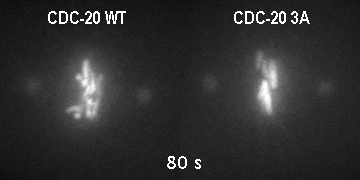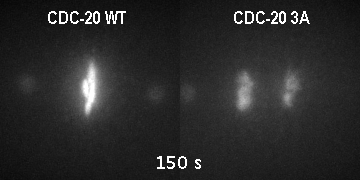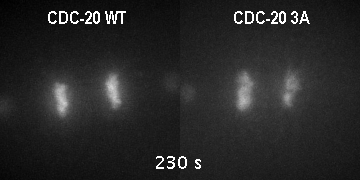
GFP::histone; gamma tubulin::GFP
Using a combination of biochemistry and live-cell imaging we are working on understanding:
1. What molecular mechanisms regulate the timing of mitosis?
2. How do these mechanisms interface with the spindle assembly checkpoint?
3. What is the importance of mitotic timing control in development?
